Copper Sulfate
Ex Tax: ج.م0egp
Availability: 1
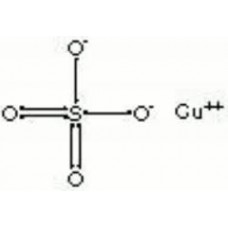
Copper Sulfate
CuSO4
Cu2SO4
Copper(II) sulfate, also known as cupric sulfate or copper sulphate, is the chemical compound with the chemical formula CuSO4. This salt exists as a series of compounds that differ in their degree of hydration. The anhydrous form is a pale green or gray-white powder, whereas the pentahydrate (CuSO4·5H2O), the most commonly encountered salt, is bright blue. Copper(II) sulfate exothermically dissolves in water to give the aquo complex [Cu(H2O)6]2+, which has octahedral molecular geometry and is paramagnetic. Other names for copper(II) sulfate are "blue vitriol" and "bluestone"
Copper sulfate is a commonly included chemical in children's chemistry sets and is often used to grow crystals in schools and in copper plating experiments.
Copper sulfate is often used to demonstrate an exothermic reaction, in which steel wool or magnesium ribbon is placed in an aqueous solution of CuSO4.
It is used in school chemistry courses to demonstrate the principle of mineral hydration.
Copper sulfate was also used in the past as an emetic.
Copper sulfate is also used to etch zinc plates for intaglio printmaking
Copper sulfate can also be used as a mordant in vegetable dyeing. It often highlights the green tints of the specific dyes.